
Plants in nature frequently perceive environmental extremes such as light stress. To maintain their physiological functions under light stress conditions, plants have developed different protection strategies that operate at morphological, anatomical and subcellular levels. The accumulation of specific light stress proteins from the ELIP (early light-induced protein) family can be considered to be a part of such protective responses. ELIPs are distant relatives of the chlorophyll a/b-binding proteins of photosystem I (PSI) and II (PSII) that accumulate only transiently in thylakoid membranes under certain physiological stress conditions. Based on predicted secondary structure and expression pattern, ELIP family members are divided into three-helix ELIPs, two-helix SEPs (stress-enhanced proteins) and one-helix OHPs (one-helix proteins) that are also called HLIPs (high light-induced proteins) in cyanobacteria and cyanophages or SCPs (small chlorophyll-binding-like proteins). Localization studies revealed that ELIPs and SEPs are located within the antenna of PSII, while OHPs form a heterodimer that accumulates in the antenna of PSI.
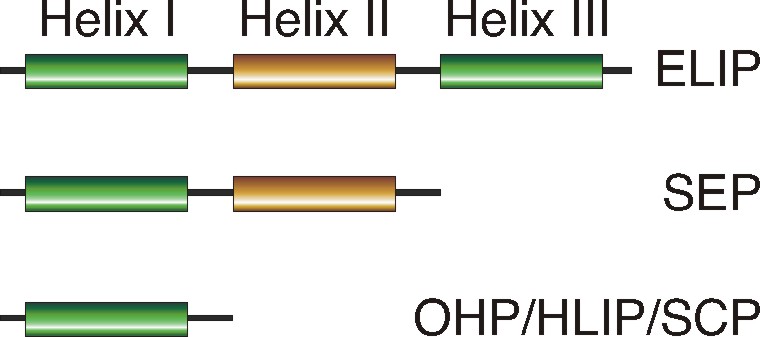
Figure 1. Schematic representation of ELIP family members. The transmembrane alpha-helices I and III are highly conserved and contain ELIP consensus motifs, while helix II is polymorphic (picture from Heddad et al. 2011).

2. The Family of Deg/HtrA serine proteases in higher plants and the cyanobacterium Synechocystis sp.PCC6803
Plant cells have evolved an extensive system of molecular chaperones, folding catalysts, and proteases that control protein quantity and quality and prevent protein damage. The protein quality control is especially important in organisms that perform oxygenic photosynthesis and generate large amounts of reactive oxygen species as byproducts that might lead to oxidative damage of proteins. The irreversibly damaged proteins or proteins dispensable to the cell are degraded by a variety of proteases. DEG proteases combine both chaperone and protease activity. DEG proteases belong to a large family of ATP-independent serine endopeptidases present in prokaryotic and eukaryotic organisms. All of these enzymes contain a protease domain of the trypsin type and carry up to four C-terminally located PDZ domains. These protein-protein interaction domains regulate the protease activity and play a role in substrate recognition. The genome of Arabidopsis thaliana encodes for 16 DEG proteases with different subcellular locations and functions. We study the role of DEG proteases in maintaining protein homeostasis in chloroplasts, mitochondria and the nucleus and their role in protein processing in the peroxisome.
We demonstrated that the chloroplast-located DEG2 protease performs the GTP-dependent primary cleavage of the photodamaged D1 protein from the photosystem II reaction center in plants. Degradation of damaged D1 and its rapid replacement by a de novo-synthesized functional copy represent an important repair mechanism of photosystem II essential for plant survival under light stress conditions.
We reported that DEG15 is a peroxisomal protease that processes peroxisomal targeting signal 2-containing proteins in vivo and in vitro. Mutant plants lacking DEG15 showed an expressed phenotype potentially linked to reduced b-oxidation of fatty acids, demonstrating for the first time the impact of protein processing on peroxisomal functions in higher eukaryotes.
Recently, we investigated biochemical properties of the nucleus-located DEG7 protease. Among the members of the DEG family, the DEG7 protease is unique since it contains two protease domains (one active and one degenerated) and four PDZ domains. We demonstrated that the presence of DEG7 is restricted to plants (including some algae) and fungi and that DEG7 from A. thaliana is located in the nucleoplasm. The DEG7 protease forms homotrimeric complexes, but in contrast to other known DEG/HtrA proteases it shows a new principle of oligomerization, where trimerization is based on interactions between the degenerated protease domains. It seems that during evolution a duplicated active protease domain degenerated and specialized in protein-protein interaction.
Finally, we investigate three DEG orthologs, HtrA, HhoA and HhoB, from the cyanobacterium Synechocystis sp. PCC6803 with the focus on the regulation of proteolytic activity, substrate specificity and formation of oligomeric complexes. We demonstrated that all three proteases can degrade unfolded model substrates, but differ in respect to cleavage sites, temperature and pH optima. All three proteases formed different homo-oligomeric complexes with and without substrate, implying mechanistic differences in comparison to each other and to the well-studied Escherichia coli orthologues DegP and DegS. Deletion of the PDZ domain decreased, but not abolished proteolytic activity of all three proteases, and prevented substrate-induced formation of complexes higher than trimers by HtrA and HhoA. In summary, biochemical characterization of HtrA, HhoA and HhoB lays the foundation for a better understanding of their overlapping, but not completely redundant stress resistance functions in Synechocystis sp. PCC 6803.

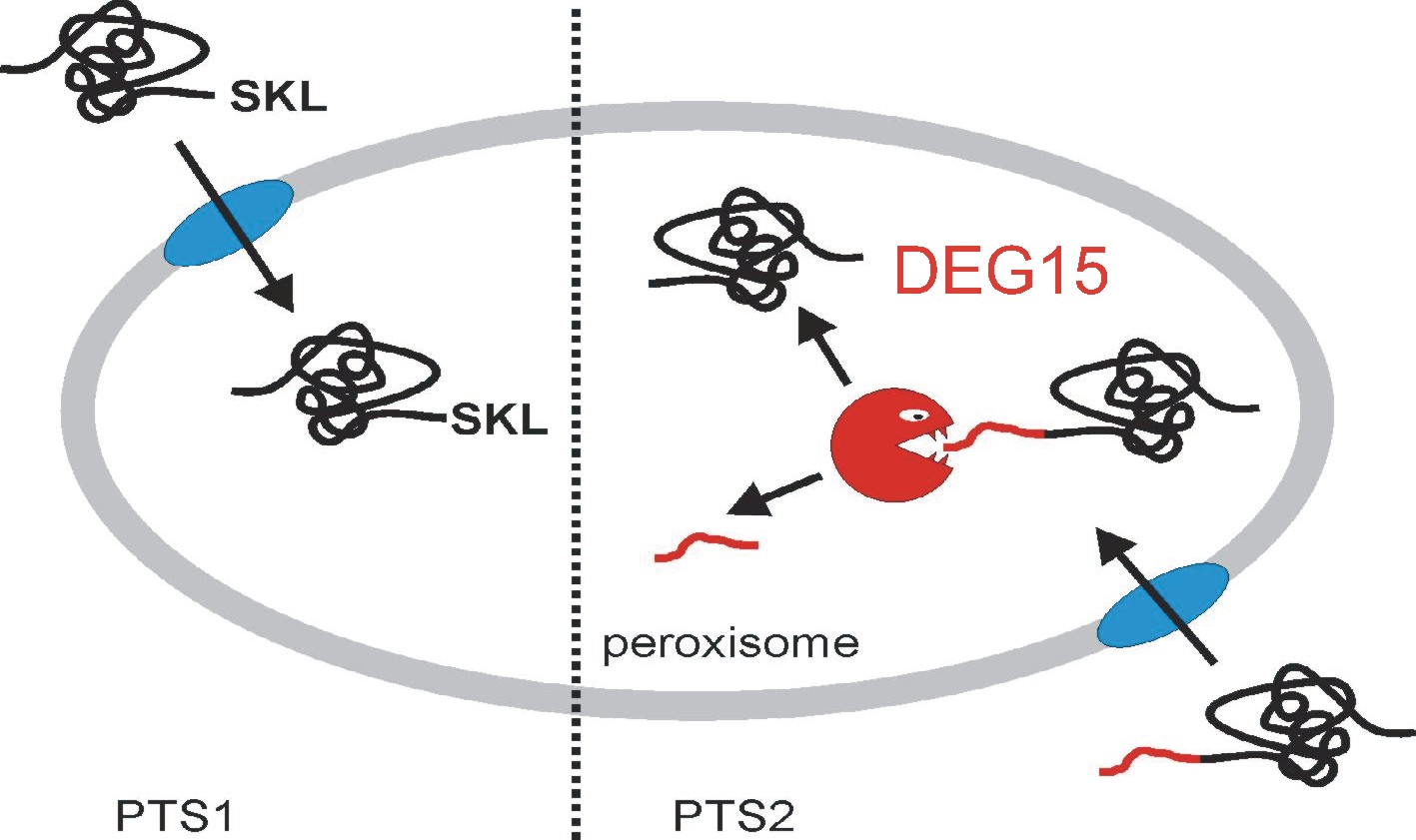
Figure 2. The role of DEG15 within the peroxisome. All peroxisomal proteins are encoded in the nucleus and are posttranslationally imported as folded proteins using two different targeting pathways. The PTS1 pathway requires the presence of a C-terminal non-cleavable tri-peptide SKL responsible for the import of the majority of peroxisomal proteins. A smaller number of proteins requires the N-terminal signal peptide PTS2 that is cleaved by the DEG15 protease at a conserved cysteine residue (picture modified from H. Schuhmann, 2008, Ph.D. thesis, University of Konstanz)