
COVID-19 vaccinations and how they work
mRNA and vector vaccines, how they work, side effects, exclusion criteria
There are currently four different COVID-19 vaccines authorized for use in Europe. According to officials and experts in Germany, all of them are equally suitable for protecting you against COVID-19 and for containing the pandemic. The facts indicate that they will prevent a majority of the severe and potentially deadly infections.
Two types of vaccines
In Germany, there are currently two kinds of vaccines available: Messenger RNA (mRNA) vaccines and viral vector vaccines.
The difference between these two types of vaccines: the mRNA vaccine directly injects the genetic blueprint for the spike protein of the coronavirus while the vector vaccine delivers the blueprint for this protein via a different, harmless virus.
Effectiveness
For all of the vaccines, the chances of getting infected with COVID-19 are significantly less after vaccination than beforehand – up to
- 70 percent lower for those vaccinated with the viral vector vaccine from Johnson & Johnson,
- 80 percent lower for those vaccinated with the same type of vaccine from AstraZeneca and
- 95 percent lower for those vaccinated with the mRNA vaccines from Biontech/Pfizer and Moderna.
This information was taken from an overview provided by the Paul-Ehrlich-Institut (PEI): Federal Institute for Vaccines and Biomedicines. The direct comparison of these percentages does not mean a lot, because they were calculated during clinical studies that did not directly compare the vaccines with each other.
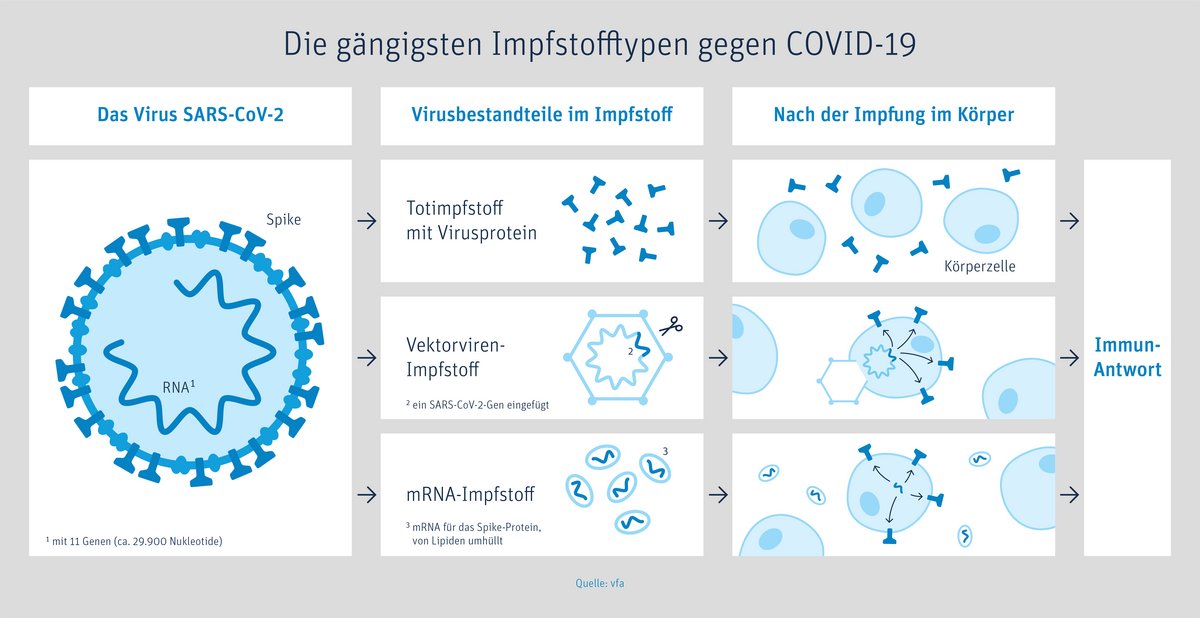
The two mRNA COVID-19 vaccines
- Comirnaty® from BioNTech/Pfizer and
- COVID-19 Vaccine Moderna® from Moderna
are nucleic acid vaccines that are based on the same technology.
The main function of the m RNA (ribonucleic acid) is to read and implement the genetic instructions for producing proteins.
The mRNA in the vaccine does not interact with or affect human DNA (genetic material). Instead, after entering cells in the body (especially muscle cells at the injection site and specific immune cells), the mRNA provides instructions for the body to produce the specific antigen (spike protein) itself. The body responds by making antibodies and immune cells that enable the body’s effective immune response.
After a few days, the body disposes of the mRNA and stops producing antigens. If a vaccinated person later comes into contact with SARS-CoV-2, his/her immune system recognizes the antigen (spike protein) and can fight off the virus/the infection effectively.
How and when does the vaccination take place?
The vaccine is injected into the muscles of the upper arm. Two doses are required. For sufficient immunization, the Robert Koch Institute (RKI)’s Standing Committee on Vaccinations (STIKO) recommends a period of 6 weeks between the first and second doses of the two mRNA COVID-19 vaccines (Comirnaty® from BioNTech/Pfizer and COVID-19 Vaccine Moderna® from Moderna). The same brand of vaccine should be used for both doses.
For persons under the age of 60 whose first dose was Vaxzevria® von AstraZeneca, STIKO recommends getting the second dose 12 weeks later using an mRNA vaccine (Comirnaty® from BioNTech/Pfizer and COVID-19 Vaccine Moderna® from Moderna).
Who should not get vaccinated?
- No vaccine has been authorized for persons under 16
- Illnesses accompanied by a fever should have completely ended
- Persons who are allergic to any of the vaccine components
- Persons who had an allergic reaction after the 1st vaccine dose
- Persons who have had a confirmed case of SARS-CoV-2 should wait at least six months after the infection. Currently it is believed that a single dose is enough to provide a longer period of immune protection
- At the moment, there is no reliable information available about vaccinations during a pregnancy/nursing period
- Other vaccinations should take place at least 14 days before or after a COVID-19 vaccination dose
- If you have a coagulation defect or take anticoagulant drugs, please discuss this beforehand with the vaccinist
Potential side effects
You may have some side effects on the arm where you got the shot or throughout the rest of your body within 2 days. They rarely last longer than 3 days.
- Pain, swelling and itching on the injection site
- Tiredness, headache, muscle pain
- Fever, chills, joint pains, nausea, swelling of the lymph nodes
These reactions are normal signs that your body is building its immune protection and they will usually go away in a few days.
Very rare side effects
- Acute facial paralysis, goes away in a few weeks, but cannot definitively be attributed to the vaccination
- Immediate allergic reaction
After the vaccination
- Waiting period of at least 15 minutes. In case of certain illnesses: 30 minutes (if recommended by the vaccinist)
- If the side effects (fever, muscle pain, tiredness) do not go away within 3 days, please consult your family doctor.
Several COVID-19 vaccines have been authorized for use. All of them are equally suitable for protecting you against COVID-19 and for containing the pandemic. The two viral vector COVID-19 vaccines
- Vaxzevria® from AstraZeneca, previously COVID-19 Vaccine AstraZeneca® and
- COVID-19 Vaccine Janssen® from Johnson & Johnson,
are subunit vaccines that are produced using modern technology.
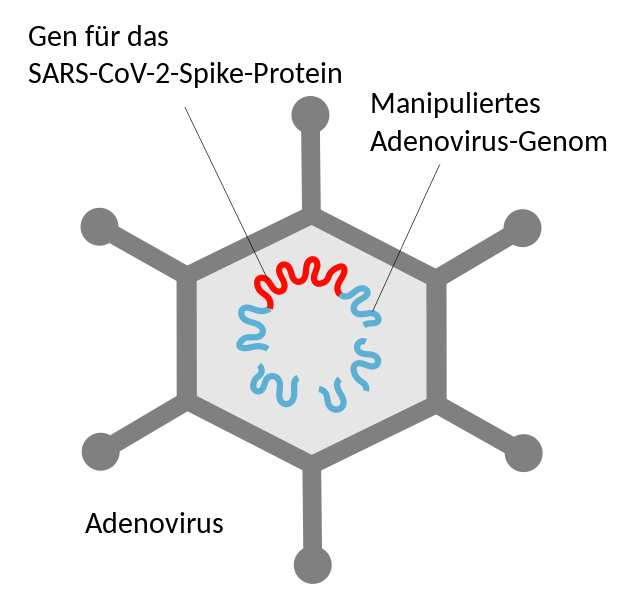
“Vector” is Latin and means “carrier”. For vector vaccines, researchers use a harmless carrier virus (not the coronavirus itself!) that cannot multiply. This vector brings only fragments of the coronavirus (spike protein on the outside of the virus) into the body.
The AstraZeneca vaccine uses a weakened chimpanzee cold virus, Johnsen & Johnsen a human adenovirus (common cold). This virus is genetically modified to contain only the blueprint for the coronavirus antigen, called the spike protein.
With the vaccine, the vector virus carrying this blueprint is delivered into a few human cells, mainly the muscle cells around the injection site. This causes these cells to produce the virus protein themselves. The body recognizes that these proteins do not belong there and triggers an immune response. The few vector viruses will soon be gone, but your immune defence is trained to respond and will kick in if you are exposed to the coronavirus.
Viral vector vaccines have been used for a while to fight dengue and ebola.
How and when does the vaccination take place?
The vaccine is injected into the muscles of the upper arm. Two doses are required for Vaxzevria® from AstraZeneca. For sufficient immunization, the Robert Koch Institute (RKI)’s Standing Committee on Vaccinations (STIKO) recommends a period of 12 weeks between the first and second doses.
At the moment, the two doses must be from the same company, except for persons under the age of 60 who were given a first dose of Vaxzevria® from AstraZeneca. For these persons, STIKO recommends getting the second dose 12 weeks later using an mRNA vaccine (Comirnaty® from BioNTech/Pfizer and COVID-19 Vaccine Moderna® from Moderna). The COVID-19 Vaccine Janssen® from Johnson & Johnson only requires one dose.
STIKO recommends AstraZeneca’s Vaxzevria® only for persons who are 60 or older. STIKO does not currently recommend this vaccine for adults under 60, since, in a few rare cases, serious illness occurred in persons under 60.
Who should not get vaccinated?
- No vaccine has been authorized for persons under 18
- STIKO currently only recommends the AstraZeneca vaccine for persons who are 60 or older
- This vaccine can be given to younger persons after they have consulted a physician
- Illnesses accompanied by a fever should have completely ended
- Persons who are allergic to any of the vaccine components
- Persons who had an allergic reaction after the 1st vaccine dose
- Persons who have had a confirmed case of SARS-CoV-2 should wait at least six months after the infection. Currently it is believed that a single dose is enough to provide a longer period of immune protection
- At the moment, there is no reliable information available about vaccinations during a pregnancy/nursing period
- Other vaccinations should take place at least 14 days before or after a COVID-19 vaccination dose
- If you have a coagulation defect or take anticoagulant drugs, please discuss this beforehand with the vaccinist
Potential side effects
You may have some side effects on the arm where you got the shot or throughout the rest of your body within 2 days. They rarely last longer than 3 days.
- Pain, swelling and itching on the injection site
- Tiredness, headache, muscle pain
- Fever, chills, joint pains, nausea, swelling of the lymph nodes
These reactions are normal signs that your body is building its immune protection and they will usually go away in a few days.
Very rare side effects
In rare cases, from 4 to 16 days after the vaccination with Vaxzevria® from AstraZeneca,
- Blood clots (thrombosis) with low blood platelet levels (thrombocytopenia), sometimes accompanied by bleeding occurred
- In a few serious cases, blood clots were observed in different and unusual places (e.g. in the brain as a cerebral venous sinus thrombosis (CVST) or abdomen with increased clotting activity) or bleeding occurred throughout the body. These side effects mainly affected persons under 60. Some of the described cases resulted in death or lasting damage
- Immediate allergic reaction
COVID-19 Vaccine Janssen® from Johnson & Johnson
In addition to the common side effects, the following have been observed:
- Rare cases of hypersensitivity and hives
- Very rare cases of immediate allergic reaction or shock
After the vaccination
- Waiting period of at least 15 minutes. In case of certain illnesses: 30 minutes (if recommended by the vaccinist)
- If the side effects (fever, muscle pain, tiredness) do not go away within 3 days, please consult your family doctor
- Please consult a physician right away if you experience difficulty breathing, chest pain, swelling of the legs, prolonged headaches, impaired vision or if, a few days after the vaccination, you observe bruising or pin-point purple, red or brown spots on the skin resulting from bleeding (petechia) outside the injection site
