Check out our feature paper in the latest edition of Inorganics!
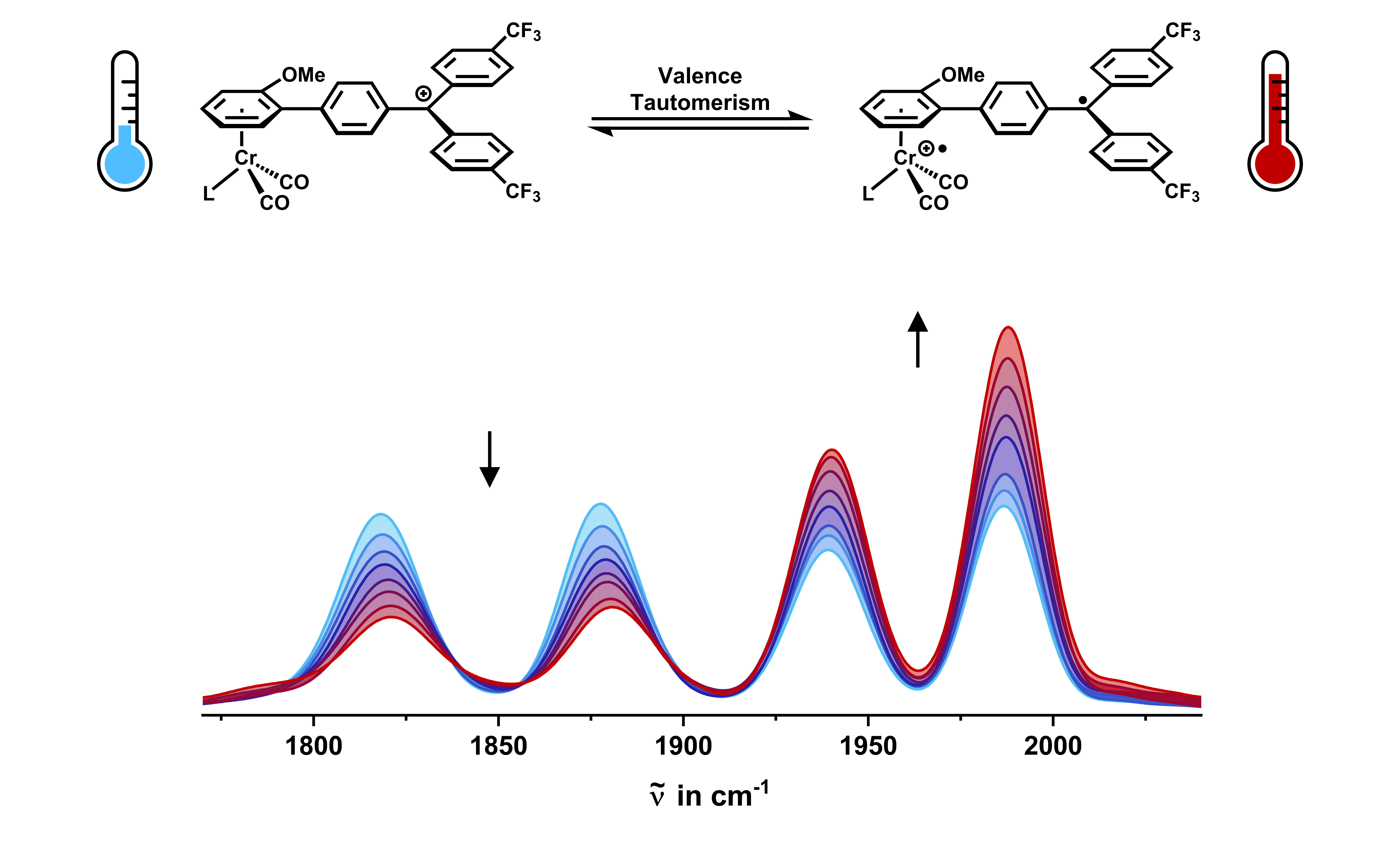
Valence tautomerism involves the coexistence of redox isomers with different charge and spin density distributions, induced by intramolecular electron transfer and stimuli like light, X-ray, or temperature variations. This study proposes chromium half-sandwich complexes as alternatives, highlighting their advantages, including easily adjustable oxidation potentials, CO ligands for infrared spectroscopy, and EPR activity at a more convenient temperature (77 K) compared to ferrocene complexes.
This study reports three novel chromium half-sandwich triarylmethylium dyads, featuring different coligands at the chromium atom. The cyclic voltammetry data for their carbinol precursors indicate that variations in L significantly impact the chromium oxidation potential. The investigation focuses on the propensity of these compounds to coexist with their paramagnetic redox isomers (valence tautomers) resulting from intramolecular electron transfer. The study reveals that valence tautomerism can be intentionally incorporated into these chromium complexes by tailoring the metal unit's oxidation potential through ligand substitution, offering potential applications in magnetoswitching despite challenges posed by low thermal stability. Studies in cyclic volammetry, IR and EPR spectroscopy and single crystal XRD await. The full open-access article can be can be found here.
