Congratulations to Larissa for her latest paper!
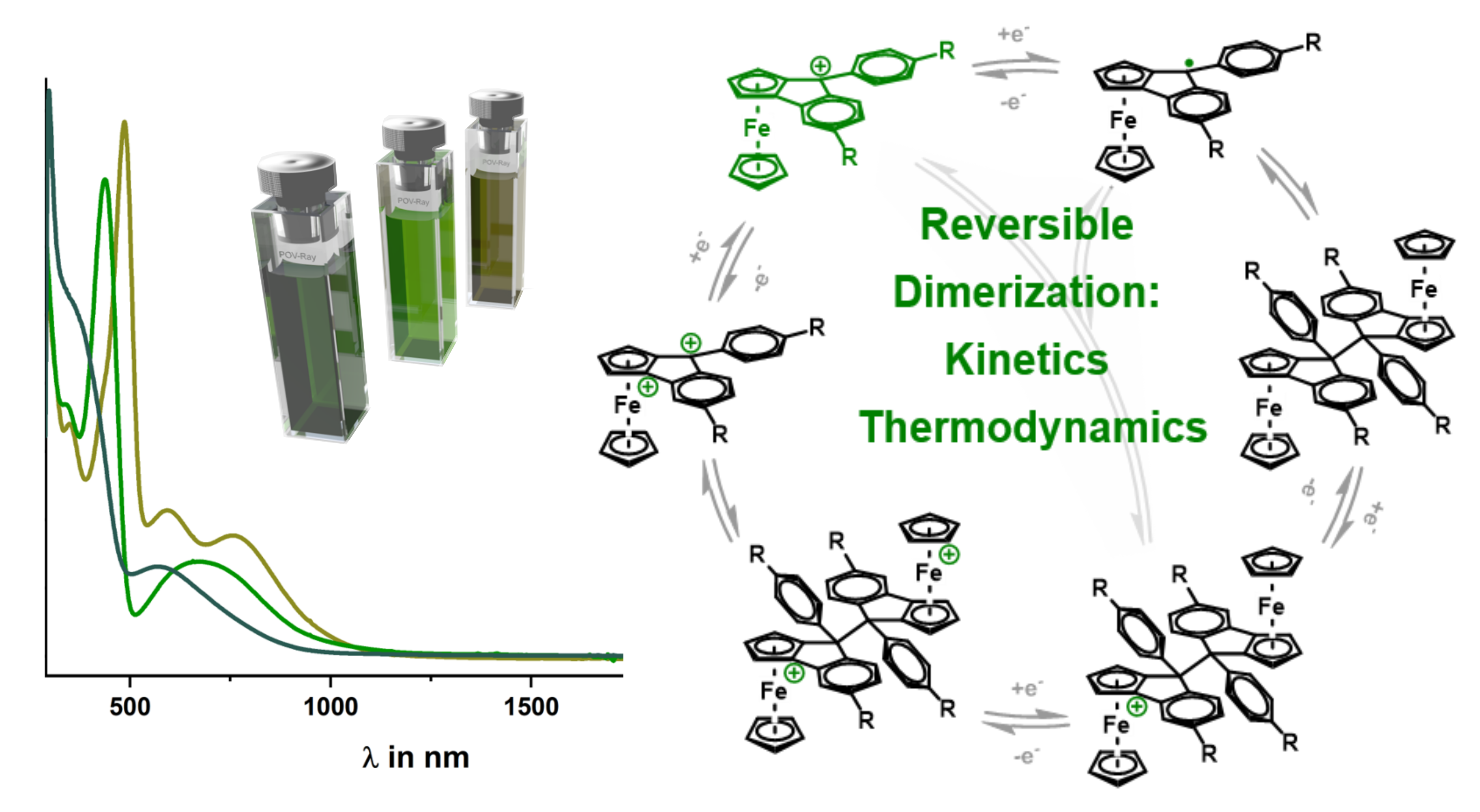
Three new ferroceno[2,3]indenylmethylium dyes (1+–3+) with 9-phenyl substitutions (OMe, Me, CF3) were studied. These dyes exist as mixtures of Rp and Sp enantiomers. The presence of a 9-phenyl group induces pyramidalization at the methyl carbon, creating a second stereocenter. The cationic complexes (1+–3+) exhibit deep green color and strong visible electronic absorption. Cyclic voltammetry and UV/vis/NIR spectroelectrochemistry revealed their electrochromic behavior. Temperature-dependent EPR spectroscopy and UV/vis/NIR spectra suggest extensive dimerization of one-electron reduced radicals (≥99.98%). Reduction of 2+ yields an isomeric dimer (2–2) that redissociates into monomers (2+) upon oxidation, demonstrating a reversible redox-induced C–C bond formation and cleavage cycle
the full article with the title "Dimerization of 9-Phenyl-ferroceno[2,3]indenylmethyl Radicals: Electrochemical and Spectroelectrochemical Studies" can be found under the following link.
