Pentafluorophenyl Groups as Remote Substituents in Ni(II)Polymerization Catalysis
Manuel Schnitte, Sophia Lipinski, Eva Schiebel, and Stefan Mecking Organometallics 2020, 39, 13-17 DOI:10.1021/acs.organomet.9b00784
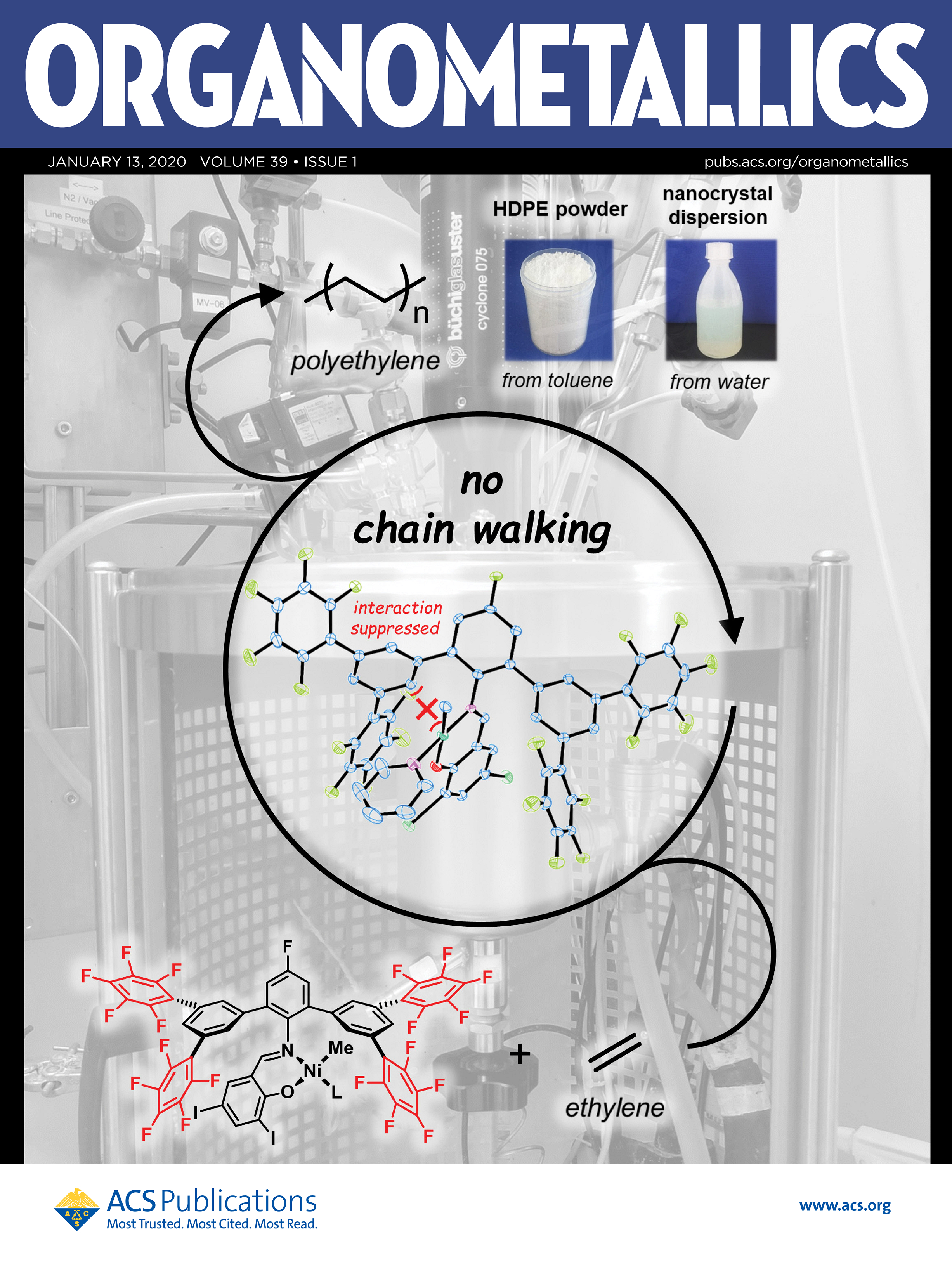
ABSTRACT: Perfluoroaromatic molecules have been studied intensively as versatile electron-poor π systems. We demonstrate the use of pentafluor-ophenyl substituents in remote positions of Ni(II) salicylaldiminato catalysts for ethylene polymerization. With their strongly electron-withdrawing character, high molecular weights of Mn>105 g/mol and simultaneously low degrees of branching are accessible due to an efficient suppression of β-hydride elimination. This can be ascribed to suppressed nickel−aryl interactions and reduced electron densities at the metal center, as concluded from X-ray crystal structure analysis and electrochemical studies. The pentafluorophenyl-substituted catalyst is stable under aqueous conditions for direct polymerization to dispersions of well-defined nanocrystals.
